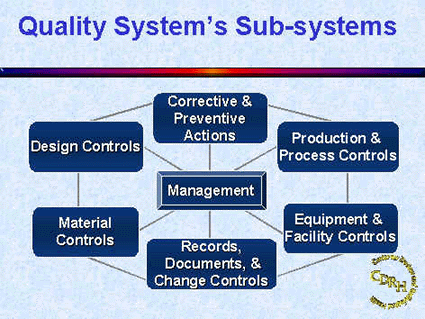
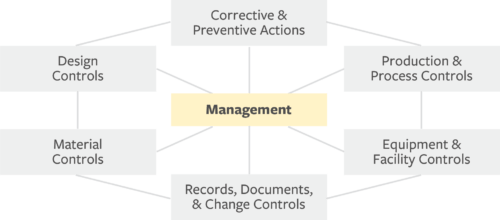
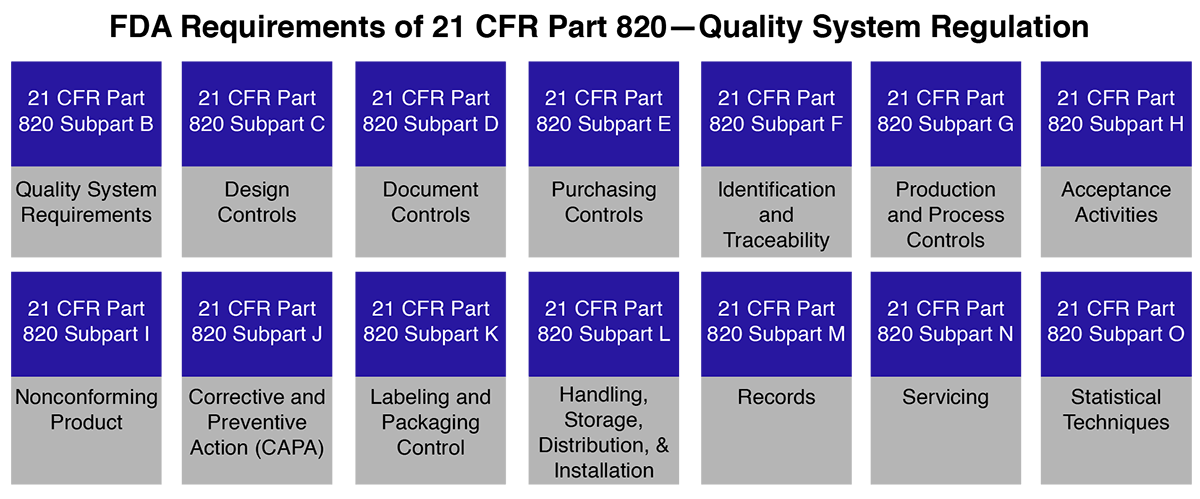
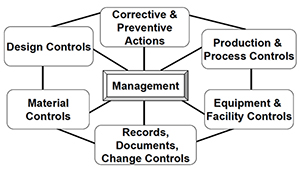
The FDA and Worldwide Quality System Requirements Guidebook for Medical Devices - Trautman, Kimberly A.: 9780873893770 - AbeBooks

FDA GMP Quality System Regulation: Handling, Storage, Distribution and Installation. : PresentationEZE

Amazon.com: FDA Quality System Regulation for Medical Devices (21 CFR Part 820): A Practitioner's Guide to Management Controls eBook : Daugherty, D: Kindle Store




%20Ultimate%20Guide%20to%2021%20CFR%20Part%20820%20%E2%80%94%20FDA%20Quality%20System%20Regulation%20(QSR)%20for%20Medical%20Devices.png?width=250&height=324&name=(cover)%20Ultimate%20Guide%20to%2021%20CFR%20Part%20820%20%E2%80%94%20FDA%20Quality%20System%20Regulation%20(QSR)%20for%20Medical%20Devices.png)